Real-time Data Saving Money and Time in Pharma Industries
INTRODUCTION
Pharmaceutical industries rely on experimental data for recognizing patterns, test theories, and understand the effectiveness of treatments. Data analytics is an evolution in a trend that has been ongoing for hundreds of years: that of human beings having ever better access to information and data. With data increasing exponentially and it getting more and more diverse, Life Science and Pharma organization face substantial challenges with data integration, data conversion, and data cleansing.
We are now in the age of “Big Data” – consisting of larger volumes, variety, and velocity of data than ever before. The capability to process and make sense of data through analytic technology brings a great opportunity for pharmaceutical companies and scientists, either by accelerating drug discovery or better understanding patient trends and behavior. Big Data is a boom for those companies looking to beat their potential. A pioneer pharma company estimates that effective big data approaches could generate up to $100 billion annually in the US healthcare system alone[2]. But to harvest the benefits, an innovative way of looking at data is required.[rml_read_more]

This article has been constructed to explain how real-time data with the appropriate data infrastructure allows the stakeholders to view data across production facilities in real-time. How such a system collects and contextualizes data from diverse sources: process, material, in-process control, quality, building management systems, and smart sensors in a trustworthy, easy-to-access platform.
WHY IS REAL-TIME DATA NEEDED IN THE PHARMA SECTORS?

- The Predictive Modelling of drugs and biological processes is a sophisticated and widespread process. But leveraging the variety of available molecular and clinical data, predictive modeling can recognize new potential-candidate molecules with a high possibility of being successfully developed into drugs that act on biological targets effectively and safely.
- In Clinical Trials, patients are identified to enroll through sources like social media rather than doctors’ visits. But, the criteria for including patients in a trial could take significantly more factors (e.g., genetic information) into consideration to target precise populations, thereby making trials smaller, shorter, less expensive, and more powerful.
- Trials Monitoring is necessary to rapidly identify safety or operational signals requiring action in real-time to avoid significant and potentially costly issues such as adverse events and unnecessary delays.
- Rigid Data Silos are difficult to exploit but electronically captured data can flow easily between functions, e.g., discovery and clinical development, as well as to external partners, for instance, physicians and contract research organizations. This easy flow is vital for driving real-time and predictive analytics that generate business value.
- Reducing Losses in Logistics as many pharmaceutical products are sensitive to temperature changes, hence necessary to distribute them through cold chain transportation, keeping the items in a chilled state during transit. In this case, temperature sensors and probes are used in the shipments that monitor temperatures change as products move through supply chains, then inform the company if any of them were outside of the optimal range for too long. It saves the patient’s life and manufacturers losses.
DATA INFRASTRUCTURE FOR BETTER CAPITAL PLANNING AND UTILIZATION IN PHARMA
A comprehensive data infrastructure removes all the hurdles in capturing, finding, converting, and organizing operational data that enables operators and engineers to model enterprise-specific operational intelligence while reducing complexity and cost. With a data infrastructure, assets can be voluntarily examined and compared, and analytics can be used for healthier long-range utilization and capital planning. A unified view empowered by a data infrastructure makes it possible to shift from scheduled maintenance to predictive or condition-based maintenance. All of this overlays the means for improved operational efficiency and higher uptime across a facility or industry.
When the industries directly benefit from developing models to support and predict asset health, process efficiency, resource management, and product quality, their regulatory reporting and compliance get simplified through end-to-end visibility.
As the stakeholders have the right info at the right time, they can examine product quality, improve capital equipment, validate environmental conditions with production, and identify and solve problems.
APPROACHES FOR ESTABLISHING STRESS-FREE OPERATIONS
Once this comprehensive data infrastructure is deployed in an organization, it serves as a foundation for building a productive and stress-free pharm manufacturing operation.
- Regulatory Compliance: The pharmaceutical industry has to follow a variety of regulations. The generation of data, the diverse ways to capture it, and the speed at which it can be communicated place a premium role on data integrity. When data fall below the standards, an organization can expect a warning letter from the regulatory authority, causing delays in operations and can even be a serious threat to the entire business.
Compliance efforts are ever-changing and becoming more proactive than reactive. The traditional three batches or reference runs are now complemented with every single run, batch and lot, including all of the parts material, process, and quality data. Data should continuously be reported as Continued Process Verification. The reliability of the data is highlighted with Data Integrity guidance’s which forms the foundation for the digitalization enabling Pharma 4.0.
- Recording and Reporting: Batch consistency is essential in pharma manufacturing. Having the right kind of automatic reporting system can help cut manufacturing time and increase production. Electronic batch recording usually requires a considerable number of pages per batch, making it costly and challenging to investigate these data to understand the potential impact on patient safety and critical quality attributes. Digital infrastructure can help by applying a rules-based system that allows for review by exclusion. We need to look at a report only when there is a violation of predetermined specifications. Such a platform can tie seamlessly into an organization’s corrective and preventive actions, further streamlining operations, reducing production time and increasing throughput.
- Knowledge Management: A particular drug takes eight to 10 years or more for commercialization from the discovery phase through clinical trials. Pharma industries are increasingly adopting a quality by design approach to gain and use more information about a molecule in real-time, speeding, and improving the process. They are incorporating measurements to their manufacturing data sensors, enhancing their knowledge of how to run equipment, and making closer observations of exactly how the material is produced inside the equipment.
- Operational Excellence: Pharmaceutical companies are maximizing their operations, improving quality, and shortening production time by using the power of data. They have reduced their production times from several weeks to a few days and in some cases, just a few hours. Energy management is optimized. Maintenance processes are streamlined via condition-based or predictive maintenance.
REAL-TIME DATA HAS ENABLED LOW-COST TREATMENT
Real-time data has also empowered the pharma companies to synchronize critical business processes, including global and site applications. The biotech firm is likely to reduce wait time for test results by 50%, batch exceptions by 70%, and batch review time by more than 70%. It is also likely to lower production costs by 80% compared with conventional biopharma operation costs for monoclonal antibody production.
DIGITAL INFRASTRUCTURE FOR REAL-TIME BATCH-TO-BATCH COMPARISONS
Conventionally different global biopharmaceutical companies could not necessarily depend on getting to a single source of the truth quickly. Batches operated with a paper system that has no batch-to-batch context, making it challenging to correlate streaming data with batch stages, and there were no batch-to-batch comparisons. The pharma manufacturing companies wanted to change that by defining specific goals:
- Assured access to reliable and error-free data.
- Empower researchers to verify experiments in just a few minutes.
- Comprehensive tribal knowledge and redundant spreadsheets in favor of scalable enterprise solutions.
- Drive a standard shift from single data points to streaming data visualization.
DATA PARADIGM FOR QUALITY ASSURANCE
Recently, top 10 pharmaceutical companies were looking to create a global Smart Solutions Store for their biologics operation to quickly enable modern and effective operations underpinned by data for Pharma 4.0 applications, use of Artificial Intelligence and Machine Learning, maintenance optimizations, use of Augmented Reality and Virtual Reality etc. at the same time as meeting regulatory demands and increase compliance.
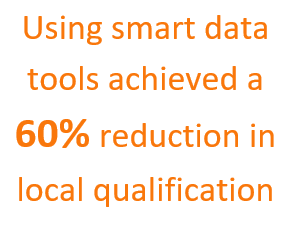
A cooperative and governance supported, the multi-site approach was used to develop and publish a business solution using smart data tools achieved a 60% reduction in local qualification efforts.
KNOWLEDGE IS AS GOOD AS THE DATA
When companies improved their collection and used real-time data by exploiting data as a key asset, over time, they came to view knowledge as a key product and they understood that their knowledge is only as good as the data it is built upon and the infrastructure that analyzes it. For these organizations, making those connections paved the way for better data, higher quality, and more efficient operations.
SMART INFRASTRUCTURE FROM ARNOWA
Smart data infrastructure provided by Arnowa captures data from sensors, equipment, control systems, and other devices and transforms it into rich, real-time insights. Anyone, from pharma engineers to executives and partners, can then use that insight to reduce costs, dramatically increase overall yield and productivity, improve quality, and make more strategic, data-driven decisions. It also helps to maintain regulatory compliance, recording, and reporting, and keep operational excellence.
OUR OFFERINGS (HARDWARE & SOFTWARE)
- Sensors: Sensors for parameters such as temperature, vibration, humidity, noise, etc., provide essential information about the process or the entire plant. They either directly measure physical values or use existing measures to indirectly calculate additional information. This information is the enabler for the implementation of advanced automatic functions, process models, as well as condition monitoring.
- Smart Utility Meters (Energy meter, gas meter, and ultrasonic water meter): This helps monitor and manage utilities.
- Wireless Data Transmission Devices (Nodes, Gateway, Edge): These devices store and transmit data to the cloud safely.
- Arnowa Application Software: It helps in the monitoring and visualization of data.
- Data Analytics Support (real intelligence): Our highly qualified and experienced intelligence team will convert the data received from the sensors into a usable form so that industries can use that data into their operational activities.
REFERENCES
[1]Kayla Matthews, 2018. 4 Ways Real-time Big Data Improves Pharma’s Bottom Line. Link: https://www.rtinsights.com/4-ways-big-data-real-time-insights-improving-pharma/
[2]Andrej Kovacevic, 2018. The Growing Importance of Big Data in the Pharmaceutical Industry. Link: https://www.smartdatacollective.com/growing-importance-big-data-pharmaceutical-industry/
[3]Wang, Y., Kung, L. and Byrd, T.A., 2018. Big data analytics: Understanding its capabilities and potential benefits for healthcare organizations. Technological Forecasting and Social Change, 126, pp.3-13.